Bioanalysis is a crucial and ever-evolving field in the lifecycle of drug development for both small molecules and biologics. Defined as the processes by which scientists determine the concentration of a drug, their metabolite, or any pharmacodynamic biomarkers within a biological fluid sample (human urine, serum, blood plasma). The multi-step process is vital for scientists to gather information about the safety and efficacy of the drug candidate they are investigating as well as being a constant benchmark to ensure proper quality assurance and control along the drug development process.
The bioanalytical methods used depend on the type of analyte you are looking for (chemical compounds or biopharmaceuticals such as monoclonal antibodies), but the fundamental principles remain the same. The intention overall is to take a clinical/pre-clinical sample and separate the desired analyte (the drug under investigation) from the matrix so that one can then take a quantitative measurement of the analyte, normally via LC-MS (small molecules) or ligand-binding assay (biologicals). Gathering this data at various stages of the drug development lifecycle can show vital information on how the drug functions in the body (pharmacology) as well as toxicology to name a few.
If we consider the stages of drug development (Figure 1), it’s pertinent to note that apart from in basic research, Bioanalysis is required to support almost all other stages in development where the clinical development (trials) can take 6-7 years alone. It has therefore been essential that upon the onset of COVID-19, pharmaceutical laboratories and CROs are able to adapt their bioanalytical operations so as to not compromise the speed at which data is collected but more importantly to ensure the data itself is of high quality.
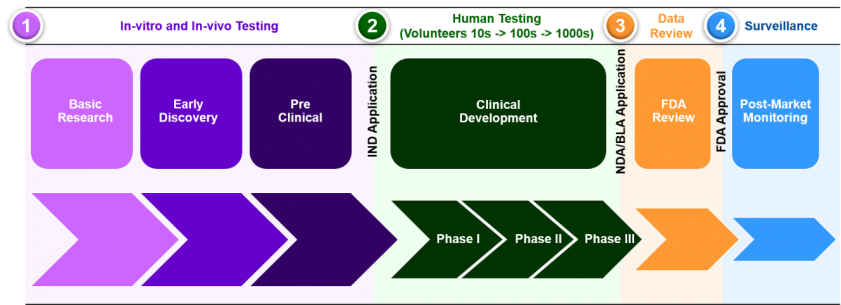
Figure 1. Representation of the Drug Development Lifecycle.
I recently spoke with Tim Sangster, Executive Director Of Bioanalysis for Celerion and Chair of the REID Bioanalytical Forum, to understand more about his thoughts on the initial challenges and how bioanalysis has adapted within Celerion and may continue to adapt as a result of COVID-19.
What were the initial challenges faced?
‘The initial challenges for Bioanalysis were in ensuring our safety of staff while operating in a laboratory environment which is normally not set up for social distancing, through adjusted shifts to 7 days a week operation, then capacity was maintained while generating appropriate social distancing.’
To build on Tim’s comments here, it’s also worth noting the importance of prioritisation. Pharmaceutical companies and CROs had to prioritise projects which require a faster turnaround such as first in human studies and deprioritise later stage study bioanalysis.
Difficulties ensuring the quantity/quality of results has not been affected?
’It is critical for regulated studies that the responsible scientist has control over the study phases and as such it was critical to be able to use online tools to allow data to be reviewed in real-time and approved so work could continue. At Celerion, the use of electronic lab notebooks significantly reduces the amount of physical paper that has to be QC's and so this allowed us to operate in the face of the pandemic highly effectively without effecting data quality of turnaround times.’
Do you feel any of the short term practices implemented will become the norm?
‘I hope that we will learn from the remote working to allow people more freedom but also the 7 days working to maximise laboratory efficiency which was not the normal business practice in many bioanalytical laboratories. While both of these can be a great benefit to the companies they can also benefit the employees by giving more flexibility in their working hours and practice.
Have you noticed more of an emphasis on the benefits/increased potential of automation?
‘I have seen a definite drive to automate more to reduce the handling of samples, while this increases efficiency it also significantly reduces the handling of samples which increases the safety of the analytical staff, particularly in the face of a novel virus which is transmitted through droplets and aerosols while being so prevalent in the environment.’
Whilst it’s clear that the importance of automation in these situations can not be understated, they also come with their own challenges. With large centrifuges and pipetting machines, an aerosol can be created and present dangers of spreading COVID-19 droplets. The significance of this means that many of the larger machines which create this effect cannot be used altogether, or must be used in a Category 2 safety cabinet.
Has there been an increased reliance on CROs? If so, why?
‘As many companies are trying to push forward existing research in their traditional therapeutic areas there has been a significant expansion of vaccine and therapeutic research to deal with the new immediate threat which we have all been faced with and this has increased the reliance on CRO's to be able to meet the requirements.’
Upon the onset of COVID-19, we originally saw the postponing of many industry clinical trials to protect patients, hospital and industry staff, and even up to now we are noticing the reluctance of many university / public hospitals to commence new trials over fears of fresh spikes, so the workload even now has not completely returned to normality. With COVID-19 looking to as if it is here to stay, Bioanalytical operations must be ready to operate again at normal capacity with no compromise on the quality of data, and external parties such as Celerion could soon be relied on more heavily to ensure no delay.